Comparing the Effects of Rest and Massage on Return to Homeostasis Following Submaximal Aerobic Exercise: a Case Study
Portia B. Resnick, MA, ATC, LMT
Department of Kinesiology and Rehabilitation Science, University of Hawaii at Mānoa, Honolulu, HI, USA.
Introduction
Postexercise massage can be used to help promote recovery from exercise on the cellular level, as well as systemically by increasing parasympathetic activity. No studies to date have been done to assess the effects of massage on postexercise metabolic changes, including excess postexercise oxygen consumption (EPOC). The purpose of this study was to compare the effects of massage recovery and resting recovery on a subject’s heart rate variability and selected metabolic effects following a submaximal treadmill exercise session.
Methods
One healthy 24-year-old female subject performed 30 minutes of submaximal treadmill exercise prior to resting or massage recovery sessions. Metabolic data were collected throughout the exercise sessions and at three 10 minute intervals postexercise. Heart rate variability was evaluated for 10 minutes after each of two 30-minute recovery sessions, either resting or massage.
Results
Heart rate returned to below resting levels (73 bpm) with 30 and 60 minutes of massage recovery (72 bpm and 63 bpm, respectively) compared to 30 and 60 minutes of resting recovery (77 bpm and 74 bpm, respectively). Heart rate variability data showed a more immediate shift to the parasympathetic state following 30 minutes of massage (1.152 LF/HF ratio) versus the 30-minute resting recovery (6.91 LF/HF ratio). It took 60 minutes of resting recovery to reach similar heart rate variability levels (1.216 LF/HF) found after 30 minutes of massage. Ventilations after 30 minutes of massage recovery averaged 7.1 bpm compared to 17.9 bpm after 30 minutes of resting recovery.
Conclusions
No differences in EPOC were observed through either the resting or massage recovery based on the metabolic data collected. Massage was used to help the subject shift into parasympathetic activity more quickly than rest alone following a submaximal exercise session.
KEYWORDS: sports massage recovery, parasympathetic activity, excess postexercise oxygen consumption (EPOC), heart rate variability, ventilatory equivalents of oxygen
INTRODUCTION
Optimal recovery allows an athlete to return to homeostasis efficiently and to perform better during subsequent training and competition sessions. Modalities such as massage, cryotherapy, hyperbaric oxygen therapy, compression garments, and stretching are suggested methods of improving recovery postexercise.(1) Massage, a mechanical manipulation of body tissues with rhythmical pressure and stroking, is often used to promote health and well-being, with the benefit of increasing parasympathetic activity and decreasing sympathetic activity in a rested state, indicating a reduced stress response.(2,3) The manual manipulation of massage is associated with increased parasympathetic activity, which indicates an improved level of homeostasis.(4) While there are no well-established techniques for evaluating parasympathetic activity, heart rate variability (HRV), the ability of the heart to modulate in response to the fine control of the parasympathetic nervous system, is often used to reflect the influence of the autonomic nervous system (ANS) on heart rate. High Frequency (HF) oscillations representing parasympathetic activity, is believed to be controlled by the vagus nerve. (5) For rested individuals, a single 40-minute session of massage is able to increase HRV in the short term, reflecting an increase in parasympathetic tone.(6)
While the manipulation of muscle and connective tissue by massage at the site of soreness has been theorized to moderate inflammation, improve blood flow, and reduce tissue stiffness, there is a lack of quality research studies on massage and its potential influence on recovery from exercise. Many of the claims made about massage lack empirical data.(2) Despite the lack of conclusive scientific evidence, massage is often provided as a part of major sporting events, prior to competition, during competition, and afterwards, in an attempt to overcome fatigue and help recovery.(2,7) A review of related literature found few studies with empirical evidence about postexercise massage on humans. In one study, postexercise massage was shown to induce changes on the cellular level in the muscles to decrease inflammation and promote recovery; however, autonomic function was not assessed.(8) In a nonhuman study, mechanical cyclical compression applied after eccentric exercise exhibited improved muscle function together with decreased damage in rabbit skeletal muscle; however, direct translation of these results cannot be made to humans.(7) While massage and exercise recovery are often associated in clinical practice, to date no study has looked at the effects of massage on metabolic responses during recovery from a submaximal bout of exercise, which may give additional insight into the effects of massage on skeletal muscle.
Following exercise, the body undergoes a postexercise elevation in resting metabolism known as excess postexercise oxygen consumption (EPOC).(9) This elevation in oxygen consumption results from an increase in energy expenditure that contributes to the overall metabolic cost of an exercise bout.(10) The magnitude and time duration of EPOC is dependent on the individual’s physical fitness level and on the intensity, duration, and mode of exercise.(11) Studies typically focus on how the mode of exercise affects EPOC, not on the how the type of recovery or any changes in the parasympathetic response may alter EPOC. In exercise recovery, the body will compensate for the increased need for oxygen by either increasing ventilations per minute or the volume of air inhaled with each breath. The metabolic variable ventilatory equivalents of oxygen (VE * VO2−1, ventilations per minute divided by oxygen uptake per minute) can be used to assess EPOC and parasympathetic recovery because efficient use of ventilatory volume has been associated with a relaxation response.(12) Adding information about metabolic data generated during a massage recovery from exercise into the current literature may provide insight into the physiological effects of massage on skeletal muscle.
Therefore, the purpose of this case study was to compare the effects of massage recovery and resting recovery on a subject’s HRV and selected metabolic effects following a submaximal treadmill exercise session. Specifically HRV, EPOC, recovery to resting heart rate, and ventilatory equivalents of oxygen were examined under conditions of complete rest or after two 30-minute massage interventions.
METHODS
Client Information
A healthy female, aged 24, with a height of 173 cm and a mass of 65 kg, consented to serve as the subject for this study. The subject was a graduate student with an above average fitness rating, based on VO2Max testing, with no known contraindications to exercise or massage. All data were collected during the Spring 2014 semester at the University of Hawai’i at Mānoa’s Human Performance Lab, as part of course requirements for graduate education in exercise physiology. As this data were acquired as an ongoing project developed for class, classroom professor oversight was used.
Assessment Measures
A Parvo Medics TrueOne 2400 (ParvoMedics, Sandy, UT, USA) metabolic cart was used to collect metabolic data through standard open-circuit indirect calorimetry procedures. Calibration was performed prior to each testing session according to the manufacturer’s instructions. The metabolic cart contains oxygen and carbon dioxide analyzers, which were calibrated to the current atmospheric conditions to determine the amount of known gases in the air. A head support and mouthpiece with a two-way, non-rebreather valve (Hans Rudolph, Kansas City, MO, USA) were connected to the metabolic cart and a standard nose clip was used to prevent movement of air into or out of the nostrils. During all metabolic data collections, the mouthpiece was placed in the subject’s mouth allowing for inhalation of room air through one valve and ensuring that the metabolic cart captured all exhalations. The metabolic cart utilized software for the analysis of the number of ventilations per minute, amount of carbon dioxide produced as a metabolic waste, and oxygen consumed in energy production in order to calculate the metabolic variables.
A Polar Pacer T31 heart rate monitor (Polar Electro, Oy, Finland) was used to collect heart rate (HR) in beats per minute (bpm) during the resting metabolic session, VO2Max test, two exercise sessions, and for the first 10-minute postexercise recovery session. Interbeat intervals were collected using a five-lead electrocardiogram (ECG) set up to record heart rate data following the resting or massage recovery sessions. This output was then used to calculate heart rate variability, as well as determine the average heart rate over that 10-minute period. Electrocardiographic data were collected using CardioCard Software Version 6.01i (Nasiff Associates, Inc., Brewerton, NY, USA) and transferred using the following protocol. The interbeat intervals (RR) were exported from WR Testworks and imported into Kubios Heart Rate Variability Software Version 2.0 (University of Kuopio, Kuopio, Finland) to assess time and frequency domain analysis. Low-level artifact correction was applied, and the sample length was set to five minutes and adjusted to find a stable pattern. Trend components were removed using a Smooth n Priors method. Frequency bands for HRV analysis were set as follows: VLF (0–0.04 Hz), LF (0.04–0.15 Hz), and HF (0.15–0.4 Hz). Interpolation of the RR series was set at 4 Hz. Window width for fast Fourier transformation was set at 512 seconds with the window overlap set at 50%. The time domain parameters addressed the magnitude of variability and provided information about the vagal (parasympathetic) modulation of the heart.(13)
Practitioner
A nationally certified and state licensed (Hawaii and New Jersey) massage therapist performed all of the massage techniques during the testing sessions. The therapist has over 10 years of experience in sports massage and has been a board of certification certified athletic trainer for 20 years. Currently the therapist is pursuing a PhD in kinesiology.
Therapeutic Intervention
The massage techniques chosen for the intervention included gliding and kneading strokes using massage cream with the intent to promote relaxation and decrease muscle tension. Swedish techniques, which are theorized to promote an increased parasympathetic response, were favored. Because all sessions were performed in a laboratory setting, massage was performed with the client clothed in shorts and a sports bra, for client comfort. A blanket was provided to cover and provide warmth to areas not being massaged. Posterior legs, neck, and upper arm, as well as the entire back were massaged for the 30-minute prone session. The 30-minute supine massage included anterior leg, the entire arm, posterior neck, face, and scalp. The two massage sessions were separated by a 10-minute session to allow for metabolic and heart rate data collection. The subject was verbally reminded to inform the therapist if at any time the treatment created discomfort. During the resting session, the subject was allowed to remain still or sleep for 30 minutes, first in the prone position and then in the supine position to maintain consistency with the massage sessions.
Procedures
Resting metabolic rate and VO2Max for the subject were established prior to the intervention sessions. Five minutes of resting metabolic data were recorded and averaged following ten minutes of supine rest. Resting values were utilized for comparison of the postexercise metabolic data collected to determine if the subject returned to baseline, and in calculation of heart rate reserve for the Karvonen formula. A modified Astrand protocol that followed ACSM guidelines for a valid outcome was used to determine the subject’s VO2Max prior to the experimental trials. The data were used to determine the subject’s heart rate maximum for use in establishing the submaximal exercise protocol.
The exercise sessions began with a 10-minute warm-up period to reach the desired heart rate zone during which no data were collected. Metabolic data collection began with 30 minutes of treadmill exercise at 80%–85% of heart rate reserve as calculated by the Karvonen formula (target heart rate = [(maximum HR − resting HR) × %intensity) + resting HR]). The speed and incline of the treadmill were continuously adjusted to maintain a comfortable pace for the subject while achieving the desired heart rate range (171–178 bpm). Exercise bouts were performed on a Quinton Medtrack ST65 treadmill. Metabolic data collected during the entire exercise portion were averaged over the 30-minute session. As previously reported by Oliveria et al.,(11) the subject completed a 24-hour fast from caffeine and alcohol as well as a 12-hour fast from food prior to EPOC data collection. Initial recovery metabolic data and HR were collected for an additional 10 minutes immediately postexercise with the subject lying in a supine position.
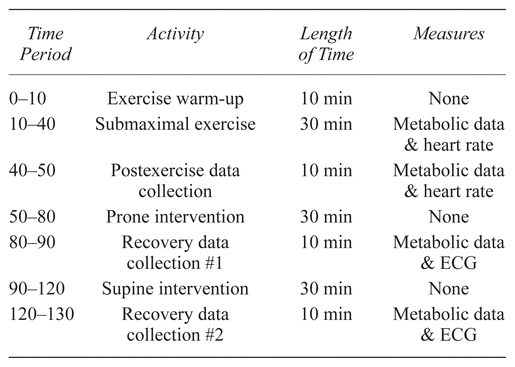
The subject completed two separate data sessions that were identical in procedure, differing only with the intervention. Data for the resting intervention were collected first with the massage intervention following two weeks later. Each session lasted approximately 130 minutes, as outlined in Table 1. Following the exercise and initial recovery data collection, the subject underwent the first 30-minute intervention, consisting of either resting recovery or massage recovery in the prone position during which no data were collected. Immediately following the first recovery intervention, the subject returned to the supine position for 10 minutes of metabolic and ECG data collection, corresponding to 40 to 50 minutes postexercise. The subject then removed the mouthpiece and remained supine for the time frame of 50 to 80 minutes postexercise for the second intervention. A final 10-minute collection for metabolic and ECG data occurred after the second recovery session, coinciding with 80 to 90 minutes postexercise. This postexercise protocol was adapted from a review by Matsuo et al.,(14) involving a 180-minute resting protocol after exercise to assess EPOC.
Table 1 Outlining the Data Collection Timeline
Each session began with a warm-up and exercise period and had two 30-minute intervention sessions. Metabolic and heart rate or ECG data were collected and averaged over the exercise and postexercise recovery and then again following each of the two recovery intervention sessions.
RESULTS
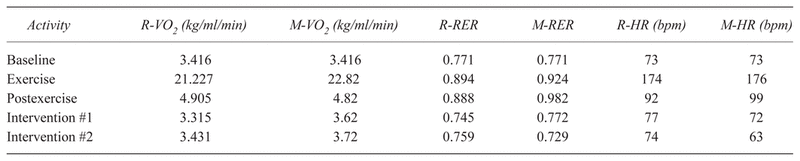
Table 2 shows the metabolic and heart rate data collected from a 24-year-old female with a VO2Max of 42 ml*kg−1*min−1 during the resting and massage recovery sessions. Postexercise VO2 for the massage recovery was higher after both the prone and supine sessions compared to the resting recovery session and did not decrease to the previously obtained resting levels. To control for the longer EPOC that occurs in the supine position, the subject followed the same position protocol for both recovery and massage trials. (9) The respiratory exchange ration (RER), the ratio of carbon dioxide produced and oxygen consumed, remained similar for both massage and resting recovery and returned to below resting levels following the second intervention session in both conditions. The average heart rate for the 30-minute exercise period in both sessions was 174 bpm, coinciding with the middle of the heart reserve estimate calculated for the subject. Following the massage recovery, heart rate decreased to just below resting levels to 72 bpm after 30 minutes of massage (40 to 50 minutes postexercise) and then fell below resting levels to 63 bpm after an additional 30 minutes of massage (80 to 90 minutes postexercise). With resting recovery, the subject’s heart rate remained elevated 40 to 50 minutes postexercise (77 bpm) and was just above baseline (74 bpm) 80 to 90 minutes postexercise.
Table 2 Recovery Metabolic Data with Rest (R) vs. Massage (M)
The subject had similar metabolic results for VO2 and RER with both interventions. Heart rate decreased to below resting levels more quickly with massage. The subject had a similar RER and VO2 in both conditions.
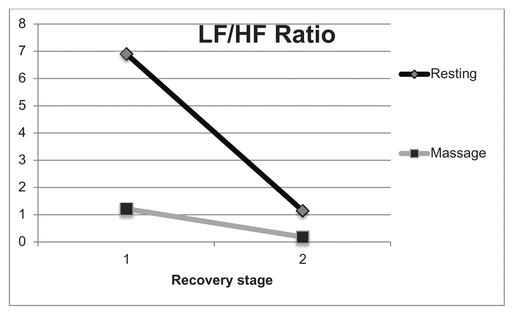
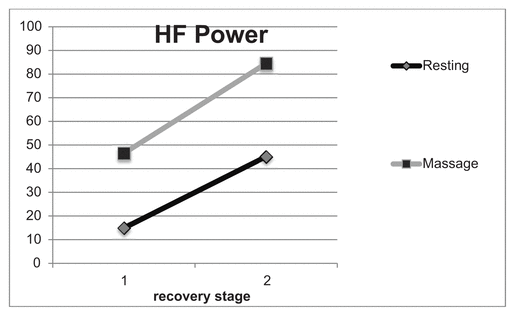
Figures 1 and 2 compare the HF and low frequency (LF) data from the heart rate variability. The subject started out with a lower LF/HF ratio following 30 minutes of massage, and that ratio continued to decrease after the second massage recovery session indicating massage resulted in a greater parasympathetic response. The ratio was higher following the first resting recovery session, but decreased after the second. Figure 2 shows an increase in HF power with the massage recovery compared to the resting recovery.
| |
|
|
|
Figure 1 Low frequency to high frequency ratio in resting vs. massage recovery 40–50 minutes (1) and 80–90 minutes (2) postexercise. The massage recover stage 1 ratio was 1.15 compared to 6.91 in the resting recovery. In stage 2, the ratio for massage was 0.18 compared to 1.22 in the resting condition. The lower ratio with the massage condition indicates a greater parasympathetic response following exercise compared to resting alone.
|
| |
|
|
|
Figure 2 Comparing high frequency, parasympathetic, power (nu) in resting recovery versus massage recovery at 40–50 minutes (1) and 80–90 minutes (2) postexercise. With massage, the subject had an increase in high frequency power (46.5 following recovery stage 1 and increasing to 84.5 after stage 2), indicating an increase in parasympathetic activity. Following resting recovery, the subject was at 14.9 and 45.1 for stages 1 and 2, respectively.
|
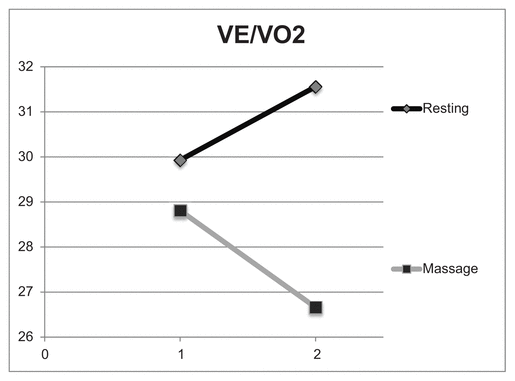
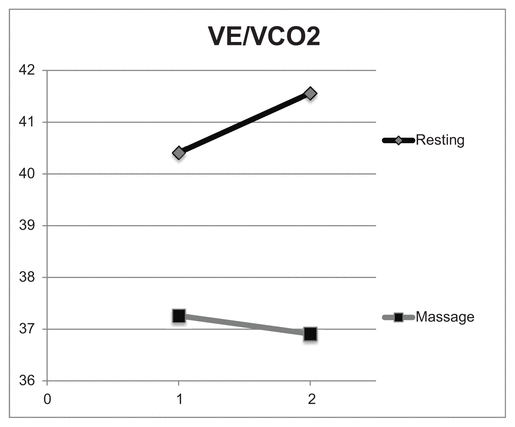
Table 3 shows the ventilatory equivalents during recovery. With massage the breaths per minute decreased after 30 minutes to seven breaths per minute (BPM), while following 30 minutes of resting recovery breaths per minute averaged almost 18 BPM. Figures 3 and 4 compare ventilatory efficiency, the slope of VE/VO2 and VE/CO2 for the resting and massage sessions. Both VE/VO2 and VE/VCO2 have a negative slope in the massage condition and a positive slope in the resting condition.
Table 3 Ventilations for Recovery
Comparing resting (R) to massage (M) values of VE, VE/VCO2, and VE/VO2, during recovery. Ventilation is in breaths per minute (BPM). Under the massage intervention, the subject had a decrease in ventilations and a decrease in VE/VO2, indicating an increased parasympathetic response.

| |
|
|
|
Figure 3 Comparison of VE/VO2 for resting versus massage recovery, 40–50 minutes (1) and 80–90 minutes (2) postexercise. These values indicate that the ratio of breaths per minute and oxygen utilized are lower postexercise in the massage condition compared to rest alone.
|
| |
|
|
|
Figure 4 Comparison of VE/VCO2 for resting versus massage recovery, 40–50 minutes (1) and 80–90 minutes (2) postexercise. These values indicate that the ratio of breaths per minute and carbon dioxide produced is lower postexercise in the massage condition compared to rest alone.
|
DISCUSSION
Since heart rate is controlled by the antagonistic work of sympathetic and parasympathetic nervous systems, the analysis of heart rate change can reflect the balance of each component of the autonomic nervous system.(3) Cardiac sympathetic nervous system activity can only affect LF components of HRV, whereas the parasympathetic nervous system can also modulate HF components with the ratio of LF power to HF power being used to assess the overall balance between the two branches of the ANS.(5,15) Following submaximal exercise, return to homeostasis was enhanced with the massage session compared to the resting session. The LF/HF ratio following the first postexercise massage recovery session was similar compared to the second postexercise resting recovery session, reflecting a quicker decrease in sympathetic control (low frequency) and an increase in parasympathetic tone (high frequency) with massage. With massage recovery, ventilations decreased at an accelerated rate compared to resting alone. Changes in breathing rate and volume can exert significant modulatory effects on the R-R interval spectra and modulate the heart variability spectra. (16) As the subject recovered from exercise, the HRV data indicated a shift to the parasympathetic state. Previous research reported a decrease in sympathetic activity with heat and massage application over time, as well as a decrease in LF/HF ratio in preterm infants undergoing massage compared to controls; however, none of these results were following an exercise session, and the massage with heat application utilized mechanical massage techniques as opposed to manual techniques.(3,17) Results comparing cool-down exercise to recovery from exercise without cool-down exercise suggested that cool-down is associated with increased HF amplitude and is closely related to the decline in the mean level of HR during recovery from exercise.(18) In this case study, a decrease in mean HR with massage recovery above and beyond that which occurred with resting recovery was observed.
Heart rate data have not been consistently reported in the literature for studies examining either EPOC or massage. Typically a change from an upright position to the supine position has been associated with a decrease in cardiac sympathetic nervous activity and an increase in vagus nerve activity. Since HR remains elevated postexercise, the rate of recovery should be quicker in the supine position compared to the upright position; however, EPOC is typically longer in the supine position.(9,19) The subject in this study recovered in a prone and supine position for both sessions and showed an accelerated decrease in average heart rate with the massage session to just below baseline after 30 minutes of massage and 10 bpm below baseline after 60 minutes of massage, coinciding with 80 to 90 minutes postexercise. Without massage, it took the full recovery period to return to slightly above resting levels. This capacity of HR to decelerate in recovery reflects parasympathetic reactivation and provides a unique perspective regarding fitness, in that recovery time for heart rate is decreased for individuals who have increased their levels of physical fitness.(20) The change in HR during recovery from exercise with massage is one possible way to reduce the work of the heart and the potential risk of cardiac events following sport activity.(4)
An interesting finding of this case study was that the VE*VO2−1 ratio was lower during the massage recovery, indicating a greater relaxation response. These results are similar to a study by Schneider and Lau(20) which resulted in significantly lower VE*VO2−1 in T’ai Chi compared to the more ballistic Wing Chun with RER values that were not significantly different. With T’ai Chi, VE*VO2−1 has been reported to reach values lower than those reported in resting states and closer to those found during self-relaxation techniques.(12) The massage recovery levels of VE*VO2−1 were also lower than those found in resting recovery alone. Because the subject’s RER was consistent with fat utilization during recovery in both conditions, the lower ratio VE*VCO2−1 in the massage recovery could be explained by the lower ventilation rate. The energy demands of the body during massage recovery were lower compared to resting recovery, with less oxygen uptake for similar RER values, making the body more efficient in the postexercise state. A close relationship has been previously demonstrated between the dynamic response of VE and VCO2 to work load changes. Comparing active and passive rest postexercise has shown a virtually identical VE*VCO2−1 relationship.(22) In contrast, both VE*VCO2−1 and VE*VO2−1 showed a decreasing trend with massage recovery and an increasing trend in resting recovery postexercise.
CONCLUSION
For this healthy, female subject, massage immediately following exercise lowered heart rate to resting levels more quickly than resting recovery alone. Massage during recovery showed a decrease in sympathetic control and an increase in parasympathetic tone in this case. Additional research using baseline HRV would be able to determine return to homeostasis. As evidenced by the VE*VO2−1 response in this case study, massage also resulted in a greater relaxation response during recovery. In addition to including more and diverse subjects, future research could focus on longer exercise duration or maximal exercise to examine the effects of massage on metabolic recovery. It was concluded that massage had a positive effect on this subject’s recovery from submaximal exercise, suggesting that massage could have a potentially positive impact on the health and recovery of other active subjects.
ACKNOWLEDGMENTS
The author wishes to recognize the following individuals for their assistance in this project. Ronald K. Hetzler, PhD, FASCM, for advising on this project, Matthew Jones and Bethany Wisthoff for contributions to the data collection sessions, Morgan Kocher for guidance with Kubios and interpretation of heart rate variability as well as all around trouble-shooting with the metabolic cart, and The Massage Therapy Foundation Executive Committee and Practitioner Case Report Contest Review Committee for their continued service to the profession and for recognizing this case study with the Gold Award, 2014
CONFLICT OF INTEREST NOTIFICATION
The author declares there are no conflicts of interest.
REFERENCES
1 Barnett A. Using recovery modalities between training sessions in elite athletes. Sports Med. 2006;36(9):781–796.

2 Weerapong P, Hume P, Kolt G. The mechanisms of massage and effects on performance, muscle recovery and injury prevention. Sports Med. 2005;35(3):235–256.

3 Lee Y, Park B, Kim S. The effects of heat and massage application on autonomic nervous system. Yonsei Med J. 2011;52(6):982–989.


4 Arroyo-Morales M, Olea N, Martinez M, Moreno-Lorenzo C, Diaz-Rodriguez L, Hidalgo-Lozano A. Effects of myofascial release after high-intensity exercise: a randomized clinical trial. J Manip Physiol Ther. 2008;31(3):217–223.

5 Heffernan KS, Kelly EE, Collier SR, Fernhall B. Cardiac autonomic modulation during recover from acute endurance versus resistance exercise. Eur J Prevent Cardiol. 2006;13(1):80–86.

6 Toro-Velasco C, Arroyo-Morales M, Fernandez-de-Las-Penas C, Cleland JA, Barrero-Hernandez FJ. Short-term effects of manual therapy on heart rate variability, mood state, and pressure pain sensitivity in patients with chronic tension-type headache: a pilot study. J Manip Physiol Ther. 2009;32(7):527–535.

7 Butterfield TA, Zhao Y, Agarwal S, Haq F, Best TM. Cyclic compressive loading facilitates recovery after eccentric exercise. Med Sci Sports Exer. 2008;40(7):1289–1296.

8 Crane JD, Ogborn DI, Cupido C, Melov S, Hubbard A, Bourgeois JM, et al. Massage therapy attenuates inflammatory signaling after exercise-induced muscle damage. Sci Translat Med. 2012;4(119):119.

9 Short K, Sedlock D. Excess postexercise oxygen consumption and recovery rate in trained and untrained subjects. J Appl Physiol. 1997;83(1):153–159.
10 Townsend J, Stout J, Morton A, Jajtner A, Gonzalez A, Wells A, et al. Excess post-exercise oxygen consumption following multiple effort sprint and moderate aerobic exercise. Kinesiol. 2013;42(1):16–21.
11 Oliveira N, Oliveira J. Excess postexercise oxygen consumption is unaffected by the resistance and aerobic exercise order in an exercise session. J Strength Cond Res. 2011;25(10):2843–2850.

12 Brown D, Mucci W, Hetzler R, Knowlton R. Cardiovascular and ventilatory responses during formalized T’ai Chi Chuan exercise. Res Q Exer Sport. 1989;60(3):246–250.

13 Task force of the European Society of Cardiology and North American Society of Pacing and Electrophysiology. Heart rate variability standards of measurement, physiological interpretation and clinical use. Eur Heart J. 1996;17:354–381.

14 Matsuo T, Ohkawara K, Seino S, Shimojo N, Yamada S, Ohshima H, et al. Cardiorespiratory fitness level correlates inversely with excess post-exercise oxygen consumption after aerobic-type interval training. BMC Res Notes. 2012;5:646.


15 Stauss HM. Heart rate variability. Am J Physiol Regul Integr Comp Physiol. 2003;285(5):R927–31.

16 De Meersman RE, Reisman SS, Daum M, Zorowitz R, Leifer M, Findley T. Influence of respiration on metabolic, hemodynamic, psychometric and R-R interval power spectral parameters. Am J Physiol–Heart Circ Physiol. 1995;269(4):H1437–H1440.
17 Smith SL, Lux R, Haley S, Slater H, Beechy J, Moyer-Mileur LJ. The effect of massage on heart rate variability in preterm infants. J Perinatol. 2013;33(1):59–64.

18 Takahashi T, Okada A, Hayano J, Tamura T. Influence of cool-down exercise on autonomic control of heart rate during recovery from dynamic exercise. Frontiers Med Biol Eng. 2001;11(4):249–259.

19 Takahashi T, Okada A, Saitoh T, Hayano J, Miyamoto Y. Difference in human cardiovascular response between upright and supine recovery from upright cycle exercise. Eur J Appl Physiol. 2000;81:233–239.

20 Cahalin LP, Forman DE, Chase P, Guazzi M, Myers J, Bensimhon D, et al. The prognostic significance of heart rate recovery is not dependent upon maximal effort in patients with heart failure. Int J Cardiol. 2013;168(2):1496–1501.

21 Schneider D, Leung R. Metabolic and cardiorespiratory responses to the performance of Wing Chun and T’ai Chi Chuan exercise. Int J Sports Med. 1991;12(3):319–323.

22 Takahashi T, Nizeki K, Miyamoto Y. Respiratory responses to passive and active recovery from exercise. Jap J Physiol. 1997;47(1):59–65.

Corresponding author: Portia B. Resnick, MA, ATC, LMT, Department of Kinesiology and Rehabilitation Science, University of Hawai’i at Mānoa, 1337 Lower Campus Road, Honolulu, HI, 96822, USA, E-mail:portia@hawaii.edu
(Return to Top)
COPYRIGHT
Published under the CreativeCommons Attribution-NonCommercial-NoDerivs 3.0 License. (
Return to Text
)
INTERNATIONAL JOURNAL OF THERAPEUTIC MASSAGE AND BODYWORK, VOLUME 9, NUMBER 1, MARCH 2016