Effects of Patterns of Pressure Application on Resting Electromyography During Massage
Langdon
Roberts
,
MA, CMT
Center for Transformational Neurophysiology, Soquel, CA, USA.
Background:
To increase the understanding of the physiological mechanisms by which massage therapy produces health benefits such as pain relief and anxiety reduction, the relationship between specific elements of massage and physiological outcomes must be addressed.
Purpose:
The effects on resting muscular activity of applying varying levels of pressure during massage were investigated.
Methods:
In this clinical crossover study, conducted in a simulated clinical setting, human subjects (
n
= 25; mean age: 34.1 years) received 3 different levels of massage pressure to the legs. A licensed therapist applied pressure to the rectus femoris in a distal-to-proximal direction. Each volunteer received the 3 levels of pressure in 2 different orders—increasing (IP) and decreasing pressures (DP)—separated by at least 4 weeks. Surface electromyography (EMG) was used to measure muscle activity levels at baseline and after each pressure level.
Results:
During the trials of IP, EMG did not vary significantly [Greenhouse–Geisser corrected analysis of variance
F
(1.71 df) = 0.30,
p
= 0.71]. During the trials of DP, EMG varied significantly [Greenhouse–Geisser corrected analysis of variance
F
(1.58 df) = 4.49,
p
= 0.03], with the largest variation, an increase of 235%, noted between baseline activity and activity after deep pressure. After application of light pressure, activity returned to baseline levels. Interestingly, the overall levels of force required to achieve subjective pressure levels as reported by the client were higher in the DP protocol than in the IP protocol
(p
< 0.02).
Conclusions:
These results suggest that the physiological response of the muscle depends on the pattern of applied pressure during massage. That finding is consistent with a mechanism by which light- or moderate-pressure massage (or a combination) may reduce the gain of spinal nociceptive reflexes. As those reflexes are elevated in chronic pain syndromes, pressure variation provides a possible mechanism for the relief of chronic pain by massage therapy.
KEYWORDS:
Massage
,
electromyography
,
nociceptors
,
psychophysiologic habituation
,
muscle tension
,
reflex
,
pain
INTRODUCTION
Over the past few decades, a substantial body of research has accumulated showing that massage therapy is effective in improving health. Chronic back pain, migraines, anxiety, hypertension, depression, and numerous other physical and psychological conditions have been shown to respond positively to massage(1–3). This type of clinical research is critical if we are to understand the potential of massage therapy as a treatment modality, and for massage to become more recognized and utilized by the mainstream medical establishment.
Despite growing evidence of the effectiveness of massage, very little has been learned about the specific physiological mechanisms that produce these benefits. Furthermore, very little scientific evidence is available about how the specific elements of massage, such as pressure levels, pacing, type of stroke, duration, and location of massage, or the psychological state of the client might contribute to the outcome of the massage. Such knowledge is essential to the creation of an objective framework for evaluating the specific massage systems or protocols that are likely to be most effective for any particular client at any particular time. To that end, the present study attempts to quantify some of the basic relationships between applied pressure and muscular response.
It has often been suggested(4) that at least some of the effects of massage are mediated by inducing muscular relaxation or alleviating muscle tension. In fact, perhaps the most common question posed to clients by massage therapists is this: “Where are you experiencing tension today?” The common assumption seems to be that maximal benefits will occur if massage is applied to the muscles that feel most tense. Subjective self-evaluations of muscle tension indicate that locally applied pressure can indeed reduce the experience of muscular tension(5).
A few attempts have been made to directly quantify the changes in muscular activity that occur during and after massage. Goldberg et al.(6) compared light and deep petrissage to the triceps surae muscle. Compared with light massage, deep massage produced a greater reduction in the H-reflex, an electrical analog of the stretch reflex. The fact that the H-reflex is reduced by massage suggests that massage therapy may produce some of its beneficial effects by reducing excitability in alpha motor neurons by 1a afferents from muscle spindles. If this is indeed the case, a large reduction in the H-reflex would seem to be desirable, because spinal hyperexcitability is associated with a variety of chronic pain syndromes(7). Although the changes in H-reflex found by Goldberg et al. did not carry over for more than about 10 s beyond the end of the massage period, a somewhat longer reduction in H-reflex amplitude (30 minutes) was found by Brouwer and Sousa de Andrade in multiple sclerosis patients after 3 minutes of stroking from occiput to coccyx(8). However, Newham and Lederman(9) found no changes in the stretch reflex in human quadriceps muscles after massage. More research would be necessary to determine whether immediate changes in the H-reflex could be related to the long-term neurological effects of massage, such as diminished response to pain(10).
Differences in pressure during massage have been shown to produce different behavioral and neurological effects. In studies by Field et al., neonates whose mothers received moderate-pressure massage during months 5 through 8 of pregnancy spent more time smiling and vocalizing, and they received better scores on the orientation, motor, excitability, and depression clusters of the Brazelton scale than did neonates whose mothers received light massage during the same period(11). In adults, moderate-pressure massage produced physiological evidence of a relaxation response that was not present in subjects who received light massage(12). Preterm infants who received moderate-pressure massage gained more weight and fared better on measures of active sleep, fussing, crying, movement, stress behavior (hiccupping), deep sleep, heart rate, and vagal tone(13).
The primary goal of the present study was to compare changes in muscular activity during massage using varying pressure levels, and to determine whether the order in which the levels of pressure were applied would make a difference in the response to specific pressure levels. Arguably, the most efficient minimally invasive method of measuring muscle activity currently available is surface electromyography (EMG). This measurement of electrical activity in the muscle is generated mainly by muscle action potentials. At activity levels likely to be found in muscles at rest, the relationship between EMG and force (“tension”) is highly correlated and essentially linear(14), and so changes in muscular activity may also reflect changes in muscular tension.
Electromyography has been effectively used to compare post-massage muscle activity for two different protocols. Naliboff and Tachiki(15) measured post-massage muscle activity produced by a Meteg Dermapoints (Daaden, Germany) massage device compared with a placebo roller. The Meteg Dermapoints roller produced a significant decrease in activity in the forearm; the other roller produced a slight increase. In the trapezius, both devices produced a small increase in activity. Cram and Vinitzky(16) compared massage on a standard massage table with massage on a body support system. They found that EMG amplitudes measured in the sitting, standing, and prone positions in 4 different muscle groups were lower after massage on the body support system than after massage on the standard table. I sought to expand those methodologies to determine whether monitoring by EMG would be an effective method for quantifying multiple responses during a multi-stage protocol.
I wanted to determine how the amount and pattern of pressure application during a massage affects levels of muscular activity in the muscles receiving the massage. Because a common approach for massage therapists is to start by applying light pressure and then to increase to deeper pressures, I was specifically interested in how the muscular response to increasing pressures (IP) compares with the response to decreasing pressures (DP). The overall quantity of pressure, as determined by the subjects, was the same in both protocols. I hypothesized that starting light and moving to IP would be more effective in reducing muscle activity than would starting deep then moving to DP.
METHODS
Subjects
Using posters at universities and grocery stores and postings on social e-mail lists in the greater Boston, MA, region, 34 subjects (29 women, 5 men) were recruited for this study. All subjects were determined to have no motor or sensory impairments and to be free of pain in both legs. No subjects were taking muscle relaxants, pain medication, or any medication prescribed for any psychological condition. All subjects were nonsmokers. All subjects were free of caffeine and alcohol for a minimum of 3 hours and free of psychoactive medications and recreational drugs for a minimum of 2 weeks before each massage session. No subjects were massage therapists. The mean age of the subjects whose data are included in the analysis was 34.1 years (range: 24 – 52 years). All protocols were approved by an internal review board of the Muscular Therapy Institute, Cambridge, MA.
Experimental Design and Procedure
In this crossover study, which was performed in a simulated clinical environment, subjects were randomly assigned to 1 of 2 groups. Each group received both the DP and IP massage protocols, but in different orders. Experimental sessions were separated by at least 4 weeks to minimize the chance of residual effects from the first protocol received. Treatment consisted of either IP or DP applied to each rectus femoris muscle.
All massage was performed using slow strokes with adjacent thumbs in a distal-to-proximal direction. Subjects listened to the same music throughout the entirety of both protocols and were asked to remain quiet and relaxed.
Pressure levels were modulated in response to feedback from the subjects, who were asked to identify levels that felt “light, but not insubstantial,” “moderate,” or “as deep as possible without causing pain or a sensation of increased muscle tension.” The IP protocol consisted of massage that started with light pressure, and then increased to moderate and finally to deep pressure. The DP protocol consisted of massage that started with deep pressure, and then decreased to moderate and finally to light pressure. For each pressure level, three strokes lasting 15 seconds each were performed. Two minutes of rest were allowed between the pressure levels. Figure 1 shows the timeline for each session.
| |

|
|
|
Figure 1.
Timeline for increasing (IP) and decreasing pressure (DP) protocols. D = period of data collection; TL = test of left leg activity; TR = test of right leg activity (data to be analyzed in future studies); I = massage therapist enters, applies pressure sensors, and gives instructions; L = application of lotion; P1 = first pressure; P2 = second pressure; P3 = third pressure.
|
Data Collection
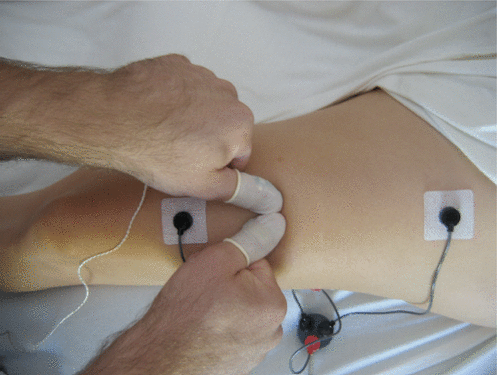
Muscular activity was assessed using EMG recordings taking with a MEDAC Sys/3 physiological monitor (NeuroDyne Medical, Cambridge, MA, USA). The EMG electrodes were attached near the musculotendinous junctions of the rectus femoris muscle of each leg (Figure 2). The ground electrodes were placed on the iliotibial tract, equidistant from the two active electrodes. This placement protocol was designed to maximize the length of the stroke, and to record data from both ends of the muscle, such that the massage occurred between the active electrodes. No qualitative difference in the magnitude of the overall relative response to massage was observed for responses measured using that placement and responses measured using a placement of 0.75 inch between electrodes at one end of the muscle.
| |
|
|
|
Figure 2.
Experimental set-up. Electromyography electrodes are attached near the musculotendinous junction of the rectus femoris. The therapist wears pressure sensors to measure force applied with adjacent thumbs.
|
The skin was prepared with alcohol, and the preamplifiers were attached using disposable electrodes and conductive gel. The MEDAC Sys/3 physiological monitor is designed to tolerate impedances of up to 200 kΩ. Skin impedance for all trials was below 100 kΩ. Heart rate was also monitored by the MEDAC Sys/3 as part of the inclusion criteria. A sudden increase in heart rate exceeding 15 bpm, sustained for at least 10 s, was considered evidence of a potential stress reaction. Force applied by the therapist’s thumbs was measured using C500 fingertip style ConTact sensors (Pressure Profile Systems, Los Angeles, CA, USA), which were pre-molded to fit the massage therapist’s thumbs and were attached using latex finger cots.
Raw EMG data were collected within a band-width of 25 – 425 Hz and were digitized at 100 Hz for analysis. Because it was impossible to tell from the data when an increase in EMG amplitude was a result of a slight movement or some other factor, I developed a system of counting repeated minima during each collection period rather than computing mean values. I consider this method to have a higher probability of producing a value that directly relates to the actual resting muscular activity levels than would any form of artifact detection and averaging over the same period. Force data from the fingertip pressure sensors was collected over the middle 10 – 12 s of each stroke and digitized at 40 Hz.
RESULTS
To avoid the possibility of interactions between massage to the first leg and massage to the second leg within a single trial, only data from the first leg treated using the protocol on any particular day (always the left leg) was used for data analysis. On blind analysis of EMG data from the 34 subjects, data from 25 subjects (22 women, 3 men) was determined to be of sufficient quality and to meet the inclusion criteria for statistical analysis.
The massage therapist chose a code to indicate which protocol was used during the collection of each data file. The code was broken only after all analyses related to inclusion and calculation of individual subject values and group means were completed. The criteria for inclusion included lack of obvious failure of the EMG equipment, at least 1 minute of data free of apparent movement artifacts during each post-massage collection period, no errors in performing the massage protocol or data collection, no sharp increases in heart rate, and no apparent signs of distress or confusion during the protocol as subjectively noted by the principal researcher or the massage therapist. The EMG data from 9 subjects were rejected because of failure to meet the foregoing criteria.
A single missing data point, belonging to the DP “light treatment” condition, was added using the mean value calculated from the other 24 subjects. Thus, the subject with the missing data point could be retained in the analysis, which was considered preferable to dropping the subject.
Mean minimum EMG baselines before application of lotion during the IP sessions (0.51 μV) and the DP sessions (0.48 μV) were not significantly different (
p
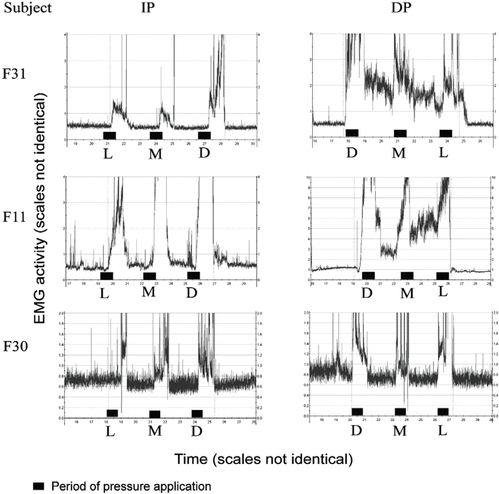
= 0.73). The EMG activity during massage strokes could not be accurately measured because of artifacts created by the massage protocol and the collection of pressure data. However, upon completion of stroke sets, muscle activity was generally elevated. During rest periods, EMG activity generally decreased compared with the levels measured immediately after periods of pressure application. No consistent pattern of decrease was noted (Figure 3). In some subjects, levels tended to drop to near or below baseline within a few seconds. In other subjects, EMG activity dropped slowly and was often still well above baseline after the 2-minute rest, when the next pressure was applied. Occasionally, EMG activity increased during a rest period.
| |
|
|
|
Figure 3.
Sample recordings of increasing (IP) and decreasing pressure (DP) sessions in 3 subjects. All 3 subjects experienced an increase in muscular activity immediately after each instance of pressure application. During the IP protocol, all 3 subjects returned to baseline levels before the end of the recording period. During the DP protocol, 2 of the 3 subjects experienced elevated activity that lasted until after the application of light pressure.
|
A repeated-measures analysis of variance (ANOVA) was conducted using the EMG data from all subjects, organized according to the actual order of treatment. The purposes for this analysis were to determine any within-subject variability and to examine the possibility of an order effect. Results indicated that EMG readings in the subjects vary as a function of time [Greenhouse–Geisser corrected ANOVA
F
(2.53 df) = 3.07,
p
= 0.04], but no significant interaction between time and order of the treatment protocols [Greenhouse–Geisser corrected ANOVA
F
(2.53 df) = 1.56,
p
= 0.22] and no between-groups effect based on order of the treatment protocols [ANOVA
F
(1 df) = 0.50,
p
= 0.49] were observed. Given the absence of evidence for an order effect, the data were then reorganized so that all DP and IP data were combined. Separate repeated-measures ANOVAs were then conducted for each treatment protocol.
Analysis of DP data indicates that EMG varied significantly across the 4 time points [Greenhouse–Geisser corrected ANOVA
F
(1.58 df) = 4.49,
p
= 0.03], an effect that did not differ according to whether a subject received the DP or the IP protocol as first treatment [Greenhouse–Geisser corrected ANOVA
F
(1.58 df) = 1.22,
p
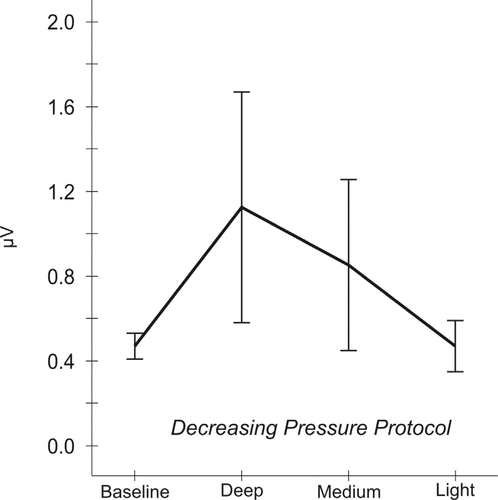
= 0.30]. The variation is represented graphically in Figure 4. In the DP condition, mean EMG levels increased from a baseline of 0.48 ± 0.03 μV (standard error of the mean) to 1.13 ± 0.27 μV after application of deep pressure. After medium and light pressures, mean levels dropped to 0.86 ± 0.20 μV and to 0.48 ± 0.06 μV respectively.
| |
|
|
|
Figure 4.
Mean electromyographic (EMG) activity responses to pressure, decreasing pressure (DP) protocol. Analysis of DP data indicates that EMG activity varies significantly across the 4 time points [Greenhouse–Geisser corrected analysis of variance
F
(1.58 df) = 4.49, p = 0.03]. Mean EMG levels are 0.48 ± 0.03
μV (standard error of the mean) at baseline, 1.13 ± 0.27 μV after deep pressure, 0.86 ± 0.20 μV after medium pressure, and 0.48 ± 0.06 μV after light pressure.
|
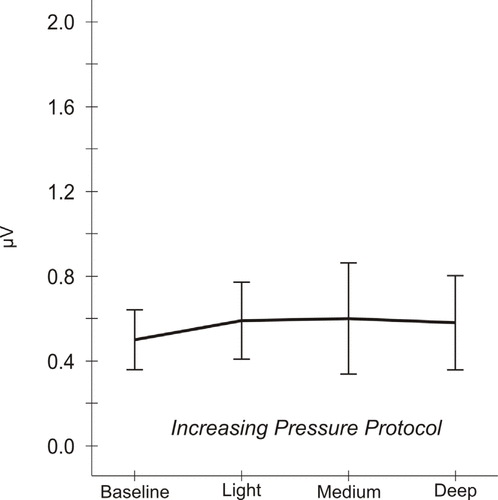
In contrast with the results for the DP protocol, analysis of IP data indicates that EMG did not vary significantly across the 4 time points [Greenhouse–Geisser corrected ANOVA
F
(1.71 df) = 0.30,
p
= 0.71]. There was also no interaction between the IP protocol and first treatment with either the DP or the IP protocol [Greenhouse–Geisser corrected ANOVA
F
(1.71 df) = 0.96,
p
= 0.38]. This overall lack of variation in the IP protocol is represented graphically in Figure 5.
| |
|
|
|
Figure 5.
Mean electromyographic (EMG) activity responses to pressure, increasing pressure (IP) protocol. Analysis of IP data indicates that EMG activity does not vary significantly across the 4 time points [Greenhouse–Geisser corrected analysis of variance
F
(1.71 df) = 0.30, p = 0.71]. Mean EMG levels are 0.51 ± 0.07 μV (standard error of the mean) at baseline, 0.60 ± 0.09 μV after light pressure, 0.61 ± 0.13 μV after medium pressure, and 0.59 ± 0.11 μV after deep pressure.
|
The EMG responses in the subjects did not appear to be uniform. Visual inspection of the data from individual subjects revealed no obvious and consistent pattern of EMG increases and decreases. Of the 25 subjects, 12 experienced an increase of at least 10% from baseline to the first pressure during the DP condition; during the IP condition, 8 of 25 experienced an increase. The largest increase observed between measurements in any given trial, 868%, occurred during the DP protocol after application of the first (deep) pressure.
Pressure Data
Because of frequent mechanical failures, pressure data from only 14 subjects (11 women, 3 men) were considered to be reliable enough for statistical treatment. Mean force levels were calculated from the voltages measured by the pressure sensors. Voltage was related to pressure using the equation
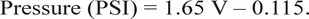
The applied pressures required to produce ratings of “light,” “moderate,” and “deep” by the subjects were higher in DP treatment than in IP treatment (Figure 6). A repeated-measures ANOVA showed that the effect was significant (
p
< 0.02). Differences between the protocols in pressures rated as “light” (Bonferroni corrected
p
< 0.15) and “moderate” (Bonferroni corrected
p
< 0.18) were borderline significant in 2-sided
t
-tests, but did not survive the Bonferroni correction.
| |
|
|
|
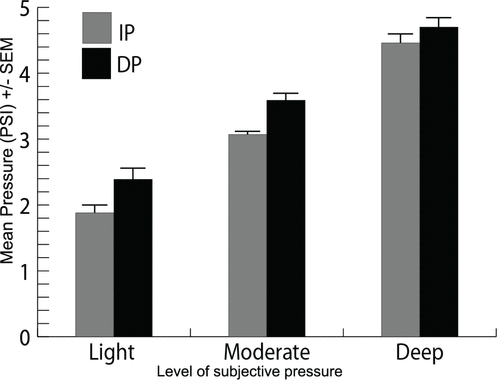
Figure 6.
Mean force required to produce various subjective levels (light, moderate, deep) of pressure, comparing increasing (IP) and decreasing pressure (DP) protocols (
n
= 14). Mean IP force levels are 1.88 ± 0.17 PSI (standard error of the mean) after light pressure, 3.07 ± 0.11 PSI after medium pressure, and 4.42 ± 0.23 PSI after deep pressure. Mean DP force levels are 2.36 ± 0.14 PSI (standard error of the mean) after light pressure, 3.53 ± 0.16 PSI after medium pressure, and 4.70 ± 0.23 PSI after deep pressure.
|
DISCUSSION
Although the DP protocol produced significant variation in muscular activity between measurements taken after different pressure levels, there was no evidence of a change in resting EMG levels after either the IP or the DP protocol. That result is consistent with findings by Naliboff and Tachiki(15) and Puustjärvi et al.(17) that massage to the trapezius did not result in a change in trapezius muscle tension. A reduction of EMG activity after massage has thus far been consistently found only in the frontalis muscle(17,18). Frontalis EMG is thought to be related to overall levels of anxiety(19). However, it would not be reasonable to extrapolate those results to massage therapy in general, given the large degree of variation in factors such as massage style, practitioner skill level, and starting levels of muscle activity and tension in the client. Furthermore, in the present study, massage was applied only to muscles that were already relaxed and not producing discomfort. That situation is infrequent in an actual massage therapy practice.
In the present study, the primary result was that, when deep pressure was applied after conditioning the muscle with light and moderate pressures (IP protocol), the rectus femoris showed no change in muscular activity, and yet when deep pressure was applied with no prior conditioning (DP protocol), the muscle responded by becoming more active. Because of the wide placement of the electrodes, it is likely that much of the measured muscular activity came from the rest of the quadriceps group, and so it is not possible to tell how much of the increase was specific to the rectus femoris(20). However, the rectus femoris acts synergistically with the rest of the quadriceps group(21), and so the contribution of multiple muscles is unlikely to have a substantial effect on the functional significance of that finding.
It is important to note that subjects were asked to allow only pressure levels that were below the pain threshold and that did not result in a conscious increase in the feeling of muscle tension. It is therefore reasonable to expect that the subjects did not notice that, on average, their muscle activity had more than doubled after the deep pressure applied during the DP protocol. That supposition is consistent with findings by Carlson et al.(22) that there was no relationship between perceived tension and EMG activity in clients with muscle pain or in pain-free subjects.
Although EMG levels remained unchanged during the IP protocol, the current results suggest that a change did occur in the level of reactivity to pressure because deep pressure in the IP protocol failed to elicit an increase in muscle activity. It is possible that the tonic stretch reflex or nociceptive reflex pathways (or both) were inhibited by application of light and moderate pressures before the deeper pressure was applied. Those reflex pathways are modified by changes in physical and mental state. The magnitude of the stretch reflex is related to task and task relevance(23). It can be modulated by factors such as static or dynamic stretching(24), postural anxiety(25), or even darkness(26). Depression of the nociceptive withdrawal reflex has previously been shown to be produced by consistent massage therapy(10), a one-time application of mechanical pressure(27), and progressive muscle relaxation(28). Leg massage has been shown to decrease pain responses to heel stick in preterm infants as assessed by the Neonatal Infant Pain Scale, which includes measures of muscle rigidity(29).
Given a high degree of variation in the response characteristics between subjects, it is possible that more than one modulating factor was influencing the results. In informal testing of the DP protocol while recording simultaneously from both the rectus femoris and the biceps femoris, only 1 in 4 subjects had a clear pattern of increased quadriceps activation and decreased hamstring activation. Because that pattern would be expected in consistent activation of the stretch reflex(30), the observed results are consistent with the idea that multiple modulating factors could be affecting levels of muscular activity. It seems likely that internal factors, such as the mental and emotional state of the subject, may have had a substantial impact on the response to a particular level of pressure. One possible determining factor of response amplitude during the massage for any individual is the relative level of parasympathetic versus sympathetic activity affecting cutaneous pathways. Cutaneous mechanoreceptors influence the magnitude of spinal reflexes and are in turn modulated by autonomic tone(31).
The overall reduction of mean response to medium and deep pressures in the IP condition compared with the DP condition may indicate that the gain of one or more spinal reflexes had been reduced. That hypothesis has important clinical implications, because spinal nociceptive reflexes are elevated in people with chronic pain(7). Alterations in the gain of nociceptive reflexes could be one mechanism by which massage may reduce pain-related behaviors and increase pain thresholds. It would be useful to perform additional studies that include measurement and comparison of both short-term and long-term indicators of nociceptive response. The findings could help to determine whether specific short-term physiological effects must be produced to achieve the long-term goal of effective pain management. Any consistent relationships found in an analysis of short-term changes in a client’s neurophysiology might then allow for the prediction of the likely long-term effectiveness of specific forms of massage or specific elements of a massage form.
In the IP protocol, the amount of force used to produce an experience of deep pressure was only slightly—not significantly—less than that in the DP protocol (although to achieve a high degree of certainty, it would be necessary to verify that result with a larger sample size), and yet the reaction of the muscle to deep pressure was much smaller in the IP protocol than in the DP protocol. It is therefore likely that an increase occurred in the ratio of applied force to motor neuron response during application of deep pressure in the IP protocol as opposed to during application of deep pressure in the DP protocol. Changes in neuronal response sensitivities are produced by changes in the ratio of pre-synaptic to post-synaptic activity levels(32). Short-term and long-term plastic changes in the central nervous system may underlie longer-term shifts in the levels of response to nociceptive activation(33,34). Therefore, after a proper warm-up period for the muscle, deep pressure may produce synaptic changes in the brain and spinal cord that lead to a reduction of both reflex activity and chronic pain. Further investigations of the relationship between response to pressure and long-term effects of massage, in clinical studies that include physiological monitoring and testing, are warranted.
When EMG levels were elevated after deep pressure, a nearly immediate reduction in activity often followed application of light pressure. That finding indicates that massage may, in fact, produce an immediate reduction in resting muscle activity if EMG levels are already elevated, although further study would be necessary to verify that the elevation of activity seen after deep pressure would not have degraded to baseline on its own with a time course similar to that of the measurements taken after light pressure in the DP protocol.
If there is a causal relationship between massage and the reduction of activity, it may be related to the feeling of “release” that is often felt during a massage, particularly when muscles are very tight to begin with. However, it is likely that mechanisms other than reduced muscular activity—such as an increase in endorphin levels(35) or a freeing of nociceptive or mechanoreceptive nerve endings(36,37) resulting from stretching of cutaneous and myofascial tissue—are generally responsible for the feelings of increased relaxation and reduced pain immediately after massage.
The levels of force that were required to produce subjective ratings of light and moderate pressure were higher when deep pressure was used first. That effect could be a result of increased levels of habituation to pressure(38–40) when higher pressures are used first. It is also possible that the order in which the different pressures were experienced affected the cognitive processes involved in the client making a subjective determination of what constituted “light,” “moderate,” or “deep” pressure. Given that increased muscular activity in the DP protocol may indicate increased muscle stiffness, it seems that more force may be required to achieve the same level of massage depth if initial pressures are deep.
The main result of the present study, that application of light and moderate pressure before application of deep pressure prevents muscle from increasing activity levels during deep pressure, has important implications for the practice of massage therapy. The approach of gradually increasing pressures, as currently practiced by many massage therapists, may have more therapeutic benefit than applying deep pressure with little or no warm-up. Pressures used in the present study were intended to be well below those that many deep-tissue practitioners use on a regular basis. It is therefore likely that even larger increases in muscular activity commonly occur during deep-tissue massage, and so it is possible that prior application of lighter pressures is even more critical in such cases. It is also evident that a client’s subjective sense of whether a muscle is relaxing into the pressure is unreliable. If a therapist’s intention is to work deeply while minimizing increases in muscle activity, EMG may turn out to be a useful tool for training practitioners to accomplish that result. Further EMG studies are warranted to address those issues.
In addition to muscular activity, there are many other physiological processes, such as autonomic nervous system activity or brain state, that could be differentially affected by varying patterns of pressure application. Monitoring these physiological changes during various massage protocols could reveal more about overall response in clients who do and who do not receive warm-up before deep pressure.
REFERENCES
1.
Field TM. Massage therapy effects.
Am Psychol.
1998; 53(12):1270–1281.

2.
Moyer CA, Rounds J, Hannum JW. A meta-analysis of massage therapy research.
Psychol Bull.
2004; 130(1):3–18.

3.
Ernst E, Pittler MH, Wider B, Boddy K. Massage therapy: is its evidence-base getting stronger?
Complement Health Pract Rev.
2007; 12(3):179–183.
4.
Goats GC. Massage—the scientific basis of an ancient art: Part 2. Physiological and therapeutic effects.
Br J Sports Med
. 1994; 28(3):153–156.

5.
Delaney JP, Leong KS, Watkins A, Brodie D. The short-term effects of myofascial trigger point massage therapy on cardiac autonomic tone in healthy subjects.
J Adv Nurs.
2002; 37(4):364–371.

6.
Goldberg J, Sullivan SJ, Seaborne DE. The effect of two intensities of massage on H-reflex amplitude.
Phys Ther.
1992; 72(6):449–457.
7.
Lidbeck J. Central hyperexcitability in chronic musculoskeletal pain: a conceptual breakthrough with multiple clinical implications.
Pain Res Manag.
2002; 7(2):81–92.
8.
Brouwer B, Sousa de Andrade V. The effects of slow stroking on spasticity in patients with multiple sclerosis: a pilot study.
Physiother Theory Pract.
1995; 11(13):13–21.

9.
Newham DJ, Lederman E. Effect of manual therapy techniques on the stretch reflex in normal human quadriceps.
Disabil Rehabil.
1997; 19(8): 326–331.

10.
Sullivan KA, Hill AE, Haussler KK. The effects of chiropractic, massage and phenylbutazone on spinal mechanical nociceptive thresholds in horses without clinical signs.
Equine Vet J.
2008; 40(1):14–20.

11.
Field T, Hernandez-Reif M, Diego M. Newborns of depressed mothers who received moderate versus light pressure massage during pregnancy.
Infant Behav Dev.
2006; 29(1):54–58.

12.
Diego MA, Field T, Sanders C, Hernandez-Reif M. Massage therapy of moderate and light pressure and vibrator effects on EEG and heart rate.
Int J Neurosci.
2004; 114(1):31–44.

13.
Field T, Diego MA, Hernandez-Reif M, Deeds O, Figuereido B. Moderate versus light pressure massage therapy leads to greater weight gain in preterm infants.
Infant Behav Dev.
2006; 29(4):574–578.

14.
Goldstein IB. Electromyography: a measure of skeletal muscle response. In: Greenfield NS, Sternbach RA, eds.
Handbook of Psychophysiology.
New York, NY: Holt, Reinhart and Winston; 1972: 329–365.
15.
Naliboff BD, Tachiki KH. Autonomic and skeletal muscle responses to nonelectrical cutaneous stimulation.
Percept Mot Skills.
1991; 72(2):575–584.

16.
Cram JR, Vinitzky I. Massage: a surface EMG comparison of the effects of a bodyCushion versus a standard massage table.
J Myofascial Therapy.
1994; 1(3):13–17.
17.
Puustjärvi K, Airaksinen O, Pöntinen PJ. The effects of massage in patients with chronic tension headache.
Acupunct Electrother Res.
1990; 15(2):159–162.
18.
Longworth JC. Psychophysiological effects of slow stroke back massage in normotensive females.
ANS Adv Nurs Sci.
1982; 4(4):44–61.
19.
Stoyva JM, Budzynski TH. Biofeedback methods in the treatment of anxiety and stress disorders. In: Lehrer PM, Woolfolk RL, eds.
Principles and Practice of Stress Management.
2nd ed. New York, NY: The Guilford Press; 1993: 263–299.
20.
Gerdle B, Karlsson S, Day S, Djupsjöbacka M. Acquisition, processing and analysis of the surface electromyogram. In: Windhorst U, Johansson H, eds.
Modern Techniques in Neuroscience.
Berlin, Germany: Springer–Verlag; 1999: 705–755.
21.
Wheeless CR III. Rectus femoris [http://../ortho/rectus_femoris]. In:
Wheeless’ Textbook of Orthopaedics.
Duke Orthopaedics presents Wheeless’ Textbook of Orthopaedics. http://www.wheelessonline.com/. Published December 14, 2008. Updated n.d. Accessed February 15, 2010.
22.
Carlson CR, Wynn KT, Edwards J, Okeson JP, Nitz AJ, Workman DE, et al. Ambulatory electromyogram activity in the upper trapezius region: patients with muscle pain vs. pain-free control subjects.
Spine (Phila Pa 1976).
1996; 21(5):595–599.
23.
Franklin DW, Wolpert DM. Specificity of reflex adaptation for task-relevant variability.
J Neurosci.
2008; 28(52):14165–14175.

24.
Kawashima N, Yano H, Ohta Y, Nakazawa K. Stretch reflex modulation during imposed static and dynamic hip movements in standing humans.
Exp Brain Res.
2006; 174(2):342–350.

25.
Sibley KM, Carpenter MG, Perry JC, Frank JS. Effects of postural anxiety on the soleus H-reflex.
Hum Mov Sci.
2007; 26(1):103–112.

26.
Safronov VA. The effect of darkness on knee-jerk reflexes.
Hum Physiol.
2009; 35(5):592–595. [From the original text: Safronov VA. The effect of darkness on knee-jerk reflexes [Russian].
Fiziol Cheloveka.
2009; 35(5):79–82]

27.
Conway BA, Knikou M. The action of plantar pressure on flexion reflex pathways in the isolated human spinal cord.
Clin Neurophysiol.
2008; 119(4):892–896.

28.
Emery CF, France CR, Harris J, Norman G, Vanarsdalen C. Effects of progressive muscle relaxation training on nociceptive flexion reflex threshold in healthy young adults: a randomized trial.
Pain.
2008; 138(2):375–379.

29.
Jain S, Kumar P, McMillan DD. Prior leg massage decreases pain responses to heel stick in preterm babies.
J Paediatr Child Health.
2006; 42(9):505–508.

30.
Mc Donough SM, Clowry GJ, Miller S, Eyre JA. Reciprocal and Renshaw (recurrent) inhibition are functional in man at birth.
Brain Res.
2001; 899(1–2):66–81.

31.
Loewenstein WR. Modulation of cutaneous mechanoreceptors by sympathetic stimulation.
J Physiol.
1956; 132(1):40–60.
32.
Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps.
Annu Rev Neurosci.
1998; 21:149–186.

33.
Bird GC, Han JS, Fu Y, Adwanikar H, Willis WD, Neugebauer V. Pain-related synaptic plasticity in spinal dorsal horn neurons: role of CGRP.
Mol Pain.
2006; 2:31.


34.
Shyu B, Vogt BA, Short-term synaptic plasticity in the nociceptive thalamic-anterior cingulate pathway.
Mol Pain.
2009; 5:51.


35.
Kaada B, Torsteinbø O. Increase of plasma beta-endorphins in connective tissue massage.
Gen Pharmacol.
1989; 20(4):487–489.
36.
Hong CZ. Current research on myofascial trigger points—pathophysiological studies.
J Musculoskelet Pain.
1999; 7(1–2):121–129.

37.
Bendtsen L, Jensen R, Olesen J. Qualitatively altered nociception in chronic myofascial pain.
Pain.
1996; 65(2–3):259–264.

38.
Dykes RW, Dancause N, Miasnikov AA, Agueev V. Rapid differential conditioning of the somatosensory evoked potential by changed patterns of brief innocuous tactile stimuli in waking rats is altered by atropine sulfate.
Brain Res.
2001; 910(1–2):74–80.

39.
Pinato G, Torre V. Coding and adaptation during mechanical stimulation in the leech nervous system.
J Physiol.
2000; 529 Pt 3:747–762.

40.
Kosek E, Hansson P. Modulatory influence on somatosensory perception from vibration and heterotopic noxious conditioning stimulation (HNCS) in fibromyalgia patients and healthy subjects.
Pain.
1997; 70(1):41–45.

Corresponding author: Langdon Roberts, Center for Transformational Neurophysiology, 4624 Soquel Drive, Soquel, California 95073 USA.
E-mail:
Langdon@neuromassage.com
(Return to Top)
COPYRIGHT
Published under the
CreativeCommons Attribution-NonCommercial-NoDerivs 3.0 License
.
CONFLICT OF INTEREST NOTIFICATION
The research reported here was funded by a grant from the Massage Therapy Foundation. Facilities and grant administration were provided by the Muscular Therapy Institute, Cambridge, MA. The massage therapist was Kilian Melloy, BA, LMT. Christopher A. Moyer, PhD, and Ann Muir Thomas, PhD, provided statistical analyses and consultation. Neurodyne Medical Corp. provided technical assistance and granted free equipment upgrades.
INTERNATIONAL JOURNAL OF THERAPEUTIC MASSAGE AND BODYWORK
, VOLUME
4
, NUMBER
1
,
MARCH 2011